Food Safety Center
Monitoring at the Food Safety Center
In charge of inspection and analysis for the Group, the Nichirei Corporation Quality Assurance Division Food Safety Center verifies that quality assurance activities at each Group company are functioning properly and preventing food-related incidents.
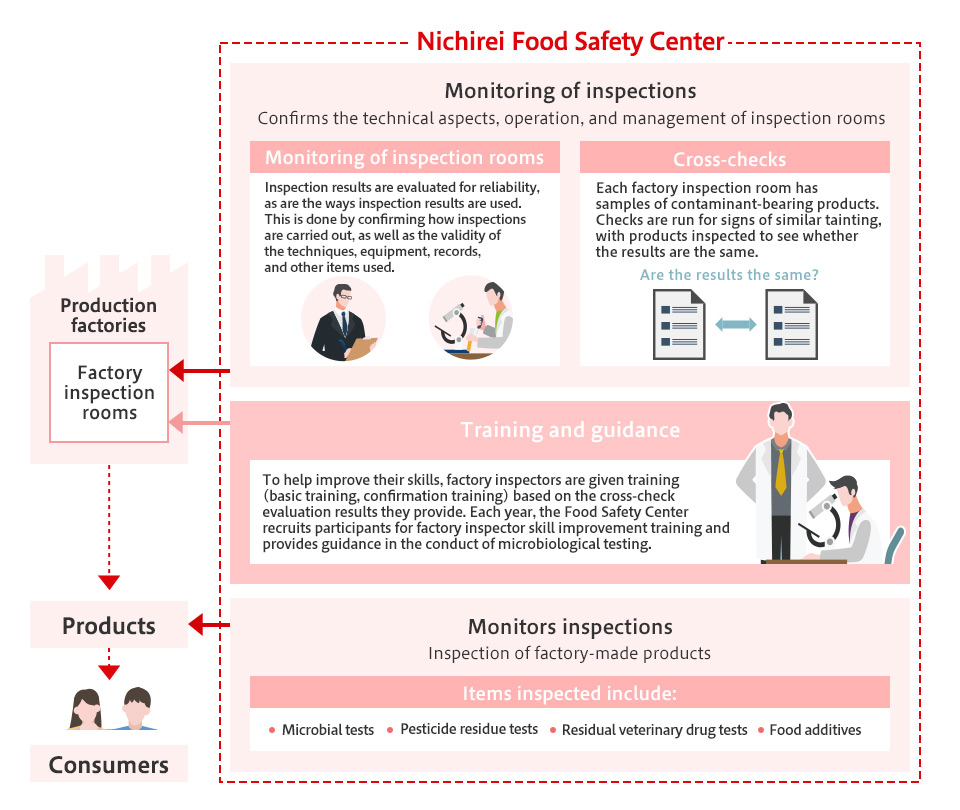
The Food Safety Center conducts product monitoring and inspections to verify that general hygiene and HACCP-compliant management efforts are being implemented at all Group factories. Domestic products are sampled and inspected immediately after production, while overseas products undergo the same processes when they are imported into Japan.
Inspections not only determine compliance with the Food Sanitation Act, they also provide feedback to Group companies on independently established standard values to help prevent future food incidents. This facilitates an examination into the appropriateness of hygiene, pesticide and medication management, with causal investigations and countermeasures incorporated into the PDCA cycle.
Additionally, to ensure that inspection results issued by factory inspection rooms are accurate, the Food Safety Center monitors laboratories and cross-checks their results. Further, the center conducts workshops for inspectors and provides technical guidance for them.

Inspection process

Hygiene inspection training
